Alzheimer’s disease, the most common case of the elderly, has plagued most people.
One of the challenges in the treatment of Alzheimer’s disease is that the delivery of therapeutic drugs to brain tissue is limited by the blood-brain barrier. The study found that MRI-guided low-intensity focused ultrasound can reversibly open the blood-brain barrier in patients with Alzheimer’s disease or other neurological disorders, including Parkinson’s disease, brain tumors, and amyotrophic lateral sclerosis.
A recent small proof-of-concept trial at the Rockefeller Institute for Neuroscience at West Virginia University showed that patients with Alzheimer’s disease who received aducanumab infusion in combination with focused ultrasound temporarily opened the blood-brain barrier significantly reduced brain amyloid beta (Aβ) load on the trial side. The research could open new doors to treatments for brain disorders.
The blood-brain barrier protects the brain from harmful substances while allowing essential nutrients to pass through. But the blood-brain barrier also prevents the delivery of therapeutic drugs to the brain, a challenge that is particularly acute when treating Alzheimer’s disease. As the world ages, the number of people with Alzheimer’s disease is increasing year by year, and its treatment options are limited, placing a heavy burden on healthcare. Aducanumab is an amyloid beta (Aβ) -binding monoclonal antibody that has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of Alzheimer’s disease, but its penetration of the blood-brain barrier is limited.
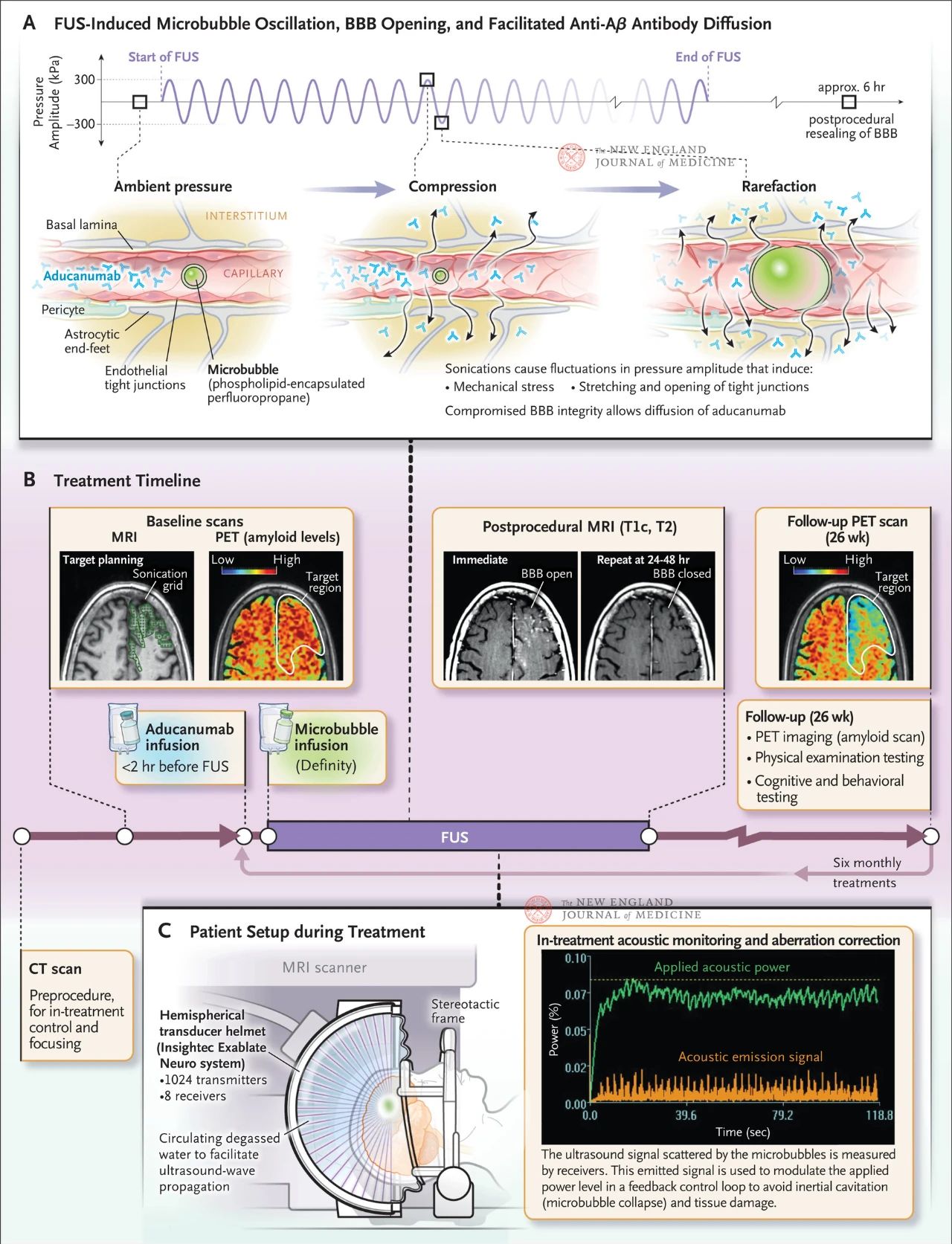
Focused ultrasound produces mechanical waves that induce oscillations between compression and dilution. When injected into the blood and exposed to the ultrasonic field, the bubbles compress and expand more than the surrounding tissue and blood. These oscillations create mechanical stress on the blood vessel wall, causing the tight connections between endothelial cells to stretch and open (Figure below). As a result, the integrity of the blood-brain barrier is compromised, allowing molecules to diffuse into the brain. The blood-brain barrier heals on its own in about six hours.
The figure shows the effect of directional ultrasound on capillary walls when micrometer-sized bubbles are present in blood vessels. Due to the high compressibility of the gas, the bubbles contract and expand more than the surrounding tissue, causing mechanical stress on the endothelial cells. This process causes tight connections to open and may also cause astrocyte endings to fall off the blood vessel wall, compromising the integrity of the blood-brain barrier and promoting antibody diffusion. In addition, endothelial cells exposed to focused ultrasound enhanced their active vacuolar transport activity and suppressed efflux pump function, thereby reducing the brain’s clearance of antibodies. Figure B shows the treatment schedule, which includes computed tomography (CT) and magnetic resonance imaging (MRI) to develop the ultrasound treatment plan, 18F-flubitaban positron emission tomography (PET) at baseline, antibody infusion before focused ultrasound treatment and microvesicular infusion during treatment, and acoustic monitoring of the microvesicular scattering ultrasound signals used to control treatment. The images obtained after focused ultrasound treatment included T1-weighted contrast-enhanced MRI, which showed that the blood-brain barrier was open in the ultrasound treated area. Images of the same area after 24 to 48 hours of focused ultrasound treatment showed complete healing of the blood-brain barrier. An 18F-flubitaban PET scan during follow-up in one of the patients 26 weeks later showed reduced Aβ levels in the brain after treatment. Figure C shows the MRI-guided focused ultrasound setup during treatment. The hemispherical transducer helmet contains more than 1,000 ultrasound sources that converge to a single focal point in the brain using real-time guidance from MRI
In 2001, focused ultrasound was first shown to induce the opening of the blood-brain barrier in animal studies, and subsequent preclinical studies have shown that focused ultrasound can enhance drug delivery and efficacy. Since then, it has been found that focused ultrasound can safely open the blood-brain barrier in patients with Alzheimer’s who are not receiving medication, and can also deliver antibodies to breast cancer brain metastases.
Microbubble delivery process
Microbubbles are an ultrasound contrast agent that is usually used to observe blood flow and blood vessels in ultrasound diagnosis. During ultrasound therapy, a phospholipid-coated non-pyrogenic bubble suspension of octafluoropropane was injected intravenously (Figure 1B). Microbubbles are highly polydispersed, with diameters ranging from less than 1 μm to more than 10 μm. Octafluoropropane is a stable gas that is not metabolized and can be excreted through the lungs. The lipid shell that wraps and stabilizes the bubbles is composed of three natural human lipids that are metabolized in a similar way to endogenous phospholipids.
Generation of focused ultrasound
Focused ultrasound is generated by a hemispherical transducer helmet that surrounds the patient’s head (Figure 1C). The helmet is equipped with 1024 independently controlled ultrasound sources, which are naturally focused in the center of the hemisphere. These ultrasound sources are driven by sinusoidal radio-frequency voltages and emit ultrasonic waves guided by magnetic resonance imaging. The patient wears a helmet and degassed water circulates around the head to facilitate ultrasound transmission. The ultrasound travels through the skin and skull to the brain target.
Changes in skull thickness and density will affect ultrasound propagation, resulting in slightly different time for ultrasound to reach the lesion. This distortion can be corrected by acquiring high-resolution computed tomography data to obtain information about skull shape, thickness, and density. A computer simulation model can calculate the compensated phase shift of each drive signal to restore the sharp focus. By controlling the phase of the RF signal, the ultrasound can be electronically focused and positioned to cover large amounts of tissue without moving the ultrasound source array. The location of the target tissue is determined by magnetic resonance imaging of the head while wearing a helmet. The target volume is filled with a three-dimensional grid of ultrasonic anchor points, which emit ultrasonic waves at each anchor point for 5-10 ms, repeated every 3 seconds. The ultrasonic power is gradually increased until the desired bubble scattering signal is detected, and then held for 120 seconds. This process is repeated on other meshes until the target volume is completely covered.
Opening the blood-brain barrier requires the amplitude of sound waves to exceed a certain threshold, beyond which the permeability of the barrier increases with increasing pressure amplitude until tissue damage occurs, manifested as erythrocyte exosmosis, bleeding, apoptosis, and necrosis, all of which are often associated with bubble collapse (called inertial cavitation). The threshold depends on the microbubble size and the shell material. By detecting and interpreting the ultrasonic signals scattered by the microbubbles, the exposure can be kept within a safe range.
After ultrasound treatment, T1-weighted MRI with contrast agent was used to determine whether the blood-brain barrier was open at the target location, and T2-weighted images were used to confirm whether extravasation or bleeding occurred. These observations provide guidance for adjusting other treatments, if necessary.
Evaluation and prospect of therapeutic effect
The researchers quantified the effect of treatment on brain Aβ load by comparing 18F-flubitaban positron emission tomography before and after treatment to assess the difference in Aβ volume between the treated area and A similar area on the opposite side. Previous research by the same team has shown that simply focusing ultrasound can slightly reduce Aβ levels. The reduction observed in this trial was even greater than in previous studies.
In the future, expanding the treatment to both sides of the brain will be critical to evaluating its efficacy in delaying disease progression. In addition, more research is needed to determine long-term safety and efficacy, and cost-effective therapeutic devices that do not rely on online MRI guidance must be developed for wider availability. Still, the findings have sparked optimism that the treatment and drugs that clear Aβ could eventually slow Alzheimer’s progression.
Post time: Jan-06-2024