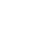Interferon is a signal secreted by the virus into the body’s descendants to activate the immune system, and is a line of defense against the virus. Type I interferons (such as alpha and beta) have been studied for decades as antiviral drugs. However, type I interferon receptors are expressed in many tissues, so the administration of type I interferon is easy to lead to an overreaction of the body’s immune response, resulting in a series of side effects. The difference is that type III interferon (λ) receptors are only expressed in epithelial tissues and certain immune cells, such as the lungs, respiratory tract, intestine, and liver, where the novel coronavirus acts, so interferon λ has fewer side effects. PEG-λ is modified by polyethylene glycol on the basis of natural interferon λ, and its circulation time in the blood is significantly longer than that of natural interferon. Several studies have shown that PEG-λ has broad-spectrum antiviral activity
As early as April 2020, scientists from the National Cancer Institute (NCI) in the United States, King’s College London in the United Kingdom and other research institutions published comments in J Exp Med recommending clinical studies using interferon λ to treat Covid-19. Raymond T. Chung, director of the Hepatobiliary Center at Massachusetts General Hospital in the United States, also announced in May that an investigator-initiated clinical trial would be conducted to evaluate the efficacy of PEG-λ against Covid-19.
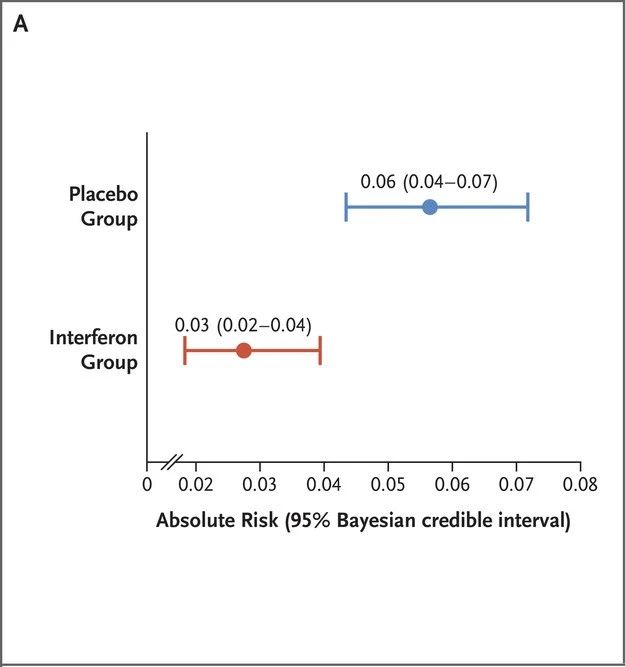
Two phase 2 clinical trials have shown that PEG-λ can significantly reduce the viral load in patients with COVID-19 [5,6]. On February 9, 2023, the New England Journal of Medicine (NEJM) published the results of a phase 3 adaptive platform trial called TOGETHER, led by Brazilian and Canadian scholars, which further evaluated the therapeutic effect of PEG-λ on COVID-19 patients [7].
Outpatients presenting with acute Covid-19 symptoms and presenting within 7 days of symptom onset received PEG-λ (single subcutaneous injection, 180 μg) or placebo (single injection or oral). The primary composite outcome was hospitalization (or referral to a tertiary hospital) or emergency department visit for Covid-19 within 28 days of randomization (observation > 6 hours).
The novel coronavirus has been mutating since the outbreak. Therefore, it is particularly important to see whether PEG-λ has curative effect on different novel coronavirus variants. The team performed subgroup analyses of the different strains of the virus that infected patients in this trial, including Omicron, Delta, Alpha, and Gamma. The results showed that PEG-λ was effective in all patients infected with these variants, and the most effective in patients infected with Omicron.
In terms of viral load, PEG-λ had a more significant therapeutic effect in patients with high baseline viral load, while no significant therapeutic effect was observed in patients with low baseline viral load. This efficacy is almost equal to Pfizer’s Paxlovid (Nematovir/Ritonavir).
It should be noted that Paxlovid is administered orally with 3 tablets twice a day for 5 days. PEG-λ, on the other hand, only requires a single subcutaneous injection to achieve the same efficacy as Paxlovid, so it has better compliance. In addition to compliance, PEG-λ has other advantages over Paxlovid. Studies have shown that Paxlovid is easy to cause drug interactions and affect the metabolism of other drugs. People with a high incidence of severe Covid-19, such as elderly patients and patients with chronic diseases, tend to take drugs for a long time, so the risk of Paxlovid in these groups is significantly higher than PEG-λ.
In addition, Paxlovid is an inhibitor that targets viral proteases. If the viral protease mutates, the drug may be ineffective. PEG-λ enhances the elimination of viruses by activating the body’s own immunity, and does not target any virus structure. Therefore, even if the virus mutates further in the future, PEG-λ is expected to maintain its efficacy.
However, the FDA said it would not authorize the emergency use of PEG-λ, much to the disappointment of the scientists involved in the study. Eiger says this may be because the study did not involve a U.S. clinical trial center, and because the trial was initiated and conducted by the researchers, not the drug companies. As a result, PEG-λ will need to invest a considerable amount of money and more time before it can be launched in the United States.
As a broad-spectrum antiviral drug, PEG-λ not only targets the novel coronavirus, it can also enhance the body’s clearance of other viral infections. PEG-λ has potential effects on influenza virus, respiratory syncytial virus and other coronaviruses. Some studies have also suggested that λ interferon drugs, if used early, can stop the virus from infecting the body. Eleanor Fish, an immunologist at the University of Toronto in Canada who was not involved in the TOGETHER study, said: “The biggest use of this type of interferon would be prophylactically, especially to protect high-risk individuals from infection during outbreaks.”
Post time: Jul-29-2023