Nosocomial pneumonia is the most common and serious nosocomial infection, of which ventilator-associated pneumonia (VAP) accounts for 40%. VAP caused by refractory pathogens is still a difficult clinical problem. For years, guidelines have recommended a range of interventions (such as targeted sedation, head elevation) to prevent VAP, but VAP occurs in up to 40% of patients with tracheal intubation, resulting in longer hospital stays, increased use of antibiotics, and death. People are always looking for more effective preventive measures.
Ventilator-associated pneumonia (VAP) is a new onset of pneumonia that develops 48 hours after tracheal intubation and is the most common and deadly nosocomial infection in the intensive care unit (ICU). The 2016 American Society of Infectious Diseases Guidelines have distinguished VAP from the definition of hospital-acquired pneumonia (HAP) (HAP only refers to pneumonia that occurs after hospitalization without a tracheal tube and is not related to mechanical ventilation; VAP is pneumonia after tracheal intubation and mechanical ventilation), and the European Society and China believe that VAP is still a special type of HAP [1-3].
In patients receiving mechanical ventilation, the incidence of VAP ranges from 9% to 27%, the mortality rate is estimated at 13%, and it can lead to increased systemic antibiotic use, prolonged mechanical ventilation, prolonged ICU stay, and increased costs [4-6]. HAP/VAP in non-immunodeficient patients is usually caused by bacterial infection, and the distribution of common pathogens and their resistance characteristics vary with region, hospital class, patient population, and antibiotic exposure, and change over time. Pseudomonas aeruginosa dominated VAP related pathogens in Europe and America, while more Acinetobacter baumannii were isolated in tertiary hospitals in China. One-third to one-half of all VAP-related deaths are directly caused by the infection, with the mortality rate of cases caused by Pseudomonas aeruginosa and acinetobacter being higher [7,8].
Due to the strong heterogeneity of VAP, the diagnostic specificity of its clinical manifestations, imaging and laboratory tests is low, and the range of differential diagnosis is wide, which makes it difficult to diagnose VAP in time. At the same time, bacterial resistance poses a serious challenge to the treatment of VAP. It is estimated that the risk of developing VAP is 3%/ day during the first 5 days of use of mechanical ventilation, 2%/ day between 5 and 10 days, and 1%/ day for the rest of the time. The peak incidence generally occurs after 7 days of ventilation, so there is a window in which infection can be prevented early [9,10]. Many studies have looked at the prevention of VAP, but despite decades of research and attempts to prevent VAP (such as avoiding intubation, preventing re-intubation, reducing sedation, raising the head of the bed by 30° to 45°, and oral care), the incidence does not appear to have decreased and the associated medical burden remains very high.
Inhaled antibiotics have been used to treat chronic airway infections since the 1940s. Because it can maximize the delivery of drugs to the target site of infection (i.e. the airway) and reduce systemic side effects, it has shown good application value in a variety of diseases. Inhaled antibiotics are now approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for use in cystic fibrosis. Inhaled antibiotics can significantly reduce bacterial load and frequency of exacerbations in bronchiectasis without increasing overall adverse events, and current guidelines have recognized them as first-line treatment for patients with pseudomonas aeruginosa infection and frequent exacerbations; Inhaled antibiotics during the perioperative period of lung transplantation can also be used as adjuvant or prophylactic drugs [11,12]. But in the 2016 U.S. VAP guidelines, experts lacked confidence in the effectiveness of adjuvant inhaled antibiotics due to the lack of large randomized controlled trials. The Phase 3 trial (INHALE) published in 2020 also failed to obtain positive results (inhale amikacin assisted intravenous antibiotics for Gram-negative bacterial infection caused by VAP patients, a double-blind, randomized, placebos controlled, phase 3 efficacy trial, a total of 807 patients, systemic medication + assisted inhalation of amikacin for 10 days).
In this context, a team led by researchers from the Regional University Hospital Centre of Tours (CHRU) in France adopted a different research strategy and conducted an investigator-initiated, multicenter, double-blind, randomized controlled efficacy trial (AMIKINHAL). Inhaled amikacin or placebo for VAP prevention was compared in 19 icus in France [13].
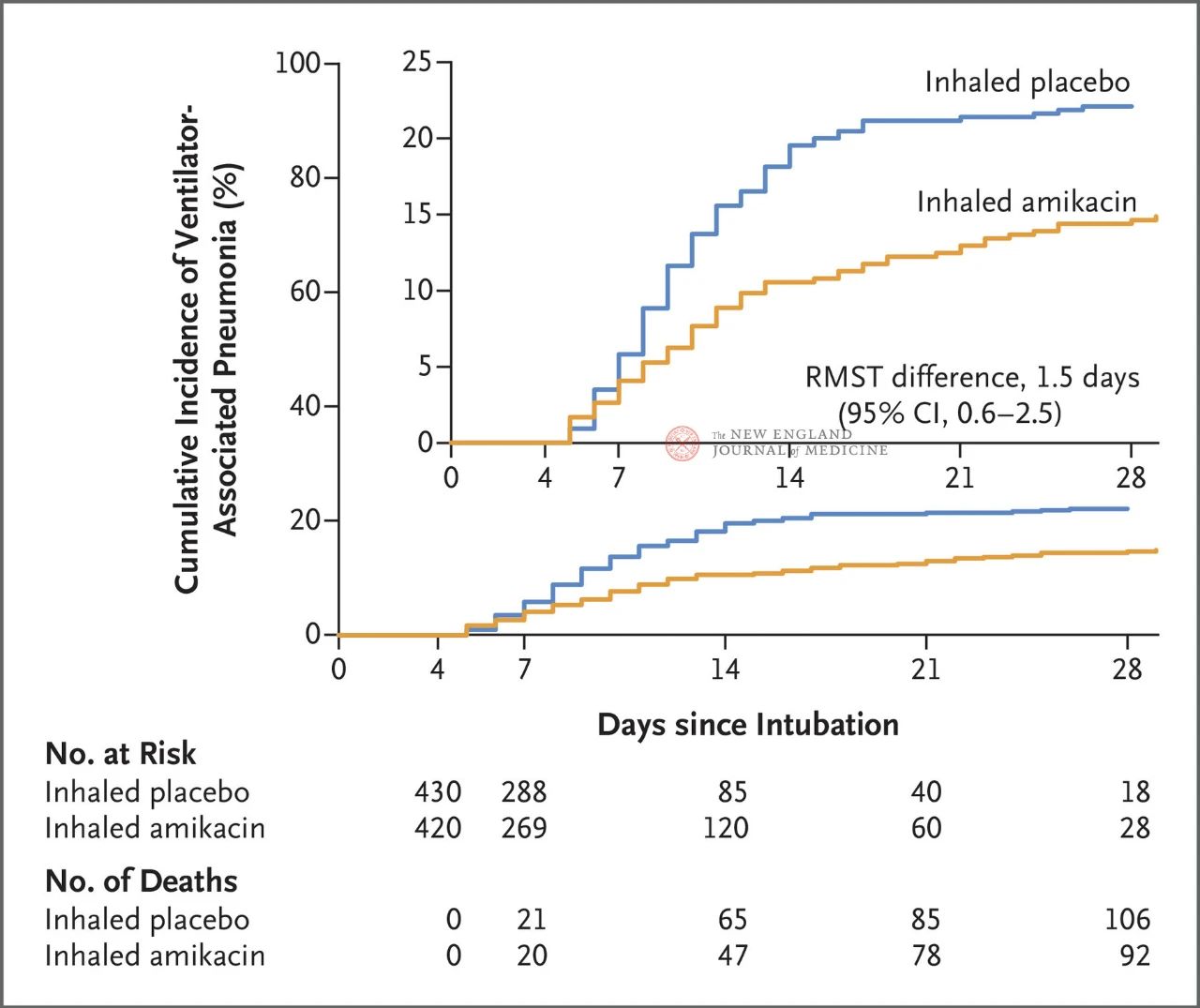
A total of 847 adult patients with invasive mechanical ventilation between 72 and 96 hours were randomly assigned 1:1 to inhalation of amikacin (N= 417,20 mg/kg ideal body weight, QD) or inhalation of placebo (N=430, 0.9% sodium chloride equivalent) for 3 days. The primary endpoint was the first episode of VAP from the beginning of randomized assignment to day 28.
Results of the trial showed that at 28 days, 62 patients (15%) in the amikacin group had developed VAP and 95 patients (22%) in the placebo group had developed VAP (the limited mean survival difference for VAP was 1.5 days; 95% CI, 0.6~2.5; P=0.004).
In terms of safety, seven patients (1.7%) in the amikacin group and four patients (0.9%) in the placebo group experienced trial-related serious adverse events. Among those who did not have acute kidney injury at randomization, 11 patients (4%) in the amikacin group and 24 patients (8%) in the placebo group had acute kidney injury at day 28 (HR, 0.47; 95% CI, 0.23~0.96).
The clinical trial had three highlights. First, in terms of study design, the AMIKINHAL trial draws on the IASIS trial (a randomized, double-blind, placebo-controlled, parallel phase 2 trial involving 143 patients). To evaluate the safety and effectiveness of amikacin – fosfomycin inhalation systemic treatment of gram-negative bacterial infection caused by VAP) and INHALE trial to end with negative results lessons learned, which focus on the prevention of VAP, and obtained relatively good results. Due to the characteristics of high mortality and long hospital stay in patients with mechanical ventilation and VAP, if amikacin inhalation can achieve significantly different results in reducing death and hospital stay in these patients, it will be more valuable for clinical practice. However, given the heterogeneity of late treatment and care in each patient and each center, there are a number of confounding factors that can interfere with the study, so it may also be difficult to obtain a positive result attributable to inhaled antibiotics. Therefore, a successful clinical study requires not only excellent study design, but also the selection of appropriate primary endpoints.
Second, although aminoglycoside antibiotics are not recommended as a single drug in various VAP guidelines, aminoglycoside antibiotics can cover common pathogens in VAP patients (including pseudomonas aeruginosa, acinetobacter, etc.), and due to their limited absorption in lung epithelial cells, high concentration at the site of infection, and low systemic toxicity. Aminoglycoside antibiotics are widely favored among inhaled antibiotics. This paper is consistent with the comprehensive estimate of the effect size of intratracheal administration of gentamicin in small samples published earlier, which jointly demonstrates the effect of inhaled aminoglycoside antibiotics in preventing VAP. It should also be noted that most of the placebo controls selected in the trials related to inhaled antibiotics are normal saline. However, considering that atomized inhalation of normal saline itself can play a certain role in diluting sputum and helping expectorant, normal saline may cause certain interference in the analysis of the study results, which should be comprehensively considered in the study.
Furthermore, local adaptation of HAP/VAP medication is important, as should antibiotic prophylaxis. At the same time, regardless of the length of intubation time, the ecology of local ICU is the most important risk factor for infection with multi-drug resistant bacteria. Therefore, the empirical treatment should refer to the microbiology data of local hospitals as much as possible, and can not blindly refer to the guidelines or the experience of tertiary hospitals. At the same time, critically ill patients requiring mechanical ventilation are often combined with multi-system diseases, and under the combined action of multiple factors such as stress state, there may also be a phenomenon of intestinal microbes crosstalk to the lungs. The high heterogeneity of diseases caused by internal and external superposition also determines that the large-scale clinical promotion of each new intervention is a long way to go.
Post time: Dec-02-2023