As career challenges, relationship problems, and social pressures mount, depression may persist. For patients treated with antidepressants for the first time, fewer than half achieve sustained remission. Guidelines on how to choose a drug after a second antidepressant treatment fails differ, suggesting that while there are many drugs available, there is little difference between them. Of these drugs, there is the most supporting evidence for increasing atypical antipsychotics.
In the latest experiment, the data of ESCAPE-TRD experiment are reported. The trial included 676 patients with depression who did not respond significantly to at least two antidepressants and were still taking selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors, such as venlafaxine or duloxetine; The purpose of the trial was to compare the efficacy of esketamine nasal spray with quetiapine sustained release. The primary endpoint was remission at 8 weeks after randomization (short-term response), and the key secondary endpoint was no recurrence at 32 weeks after remission at 8 weeks.
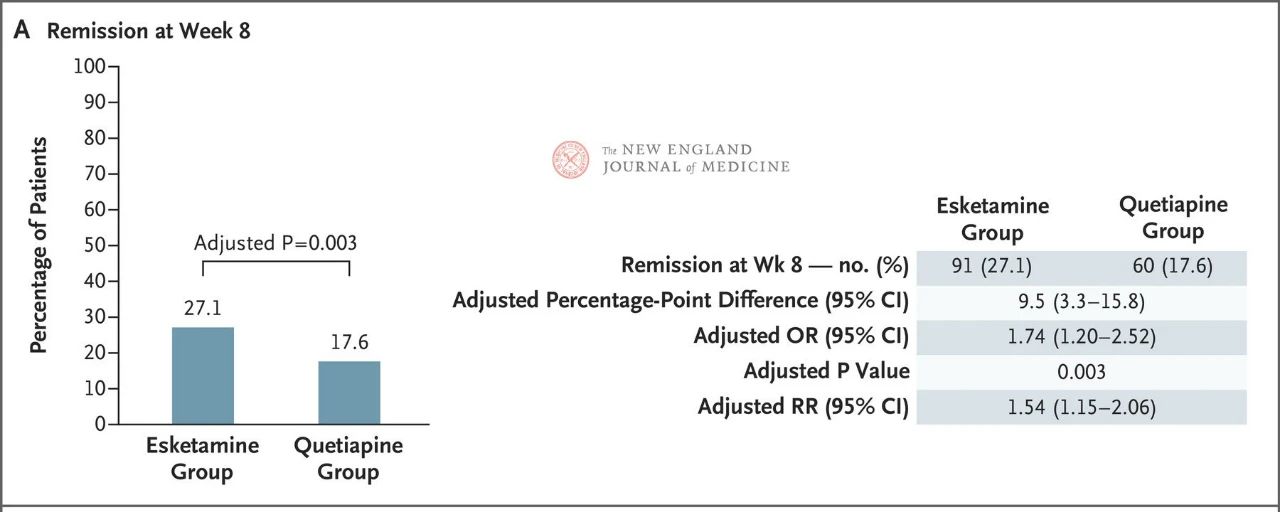
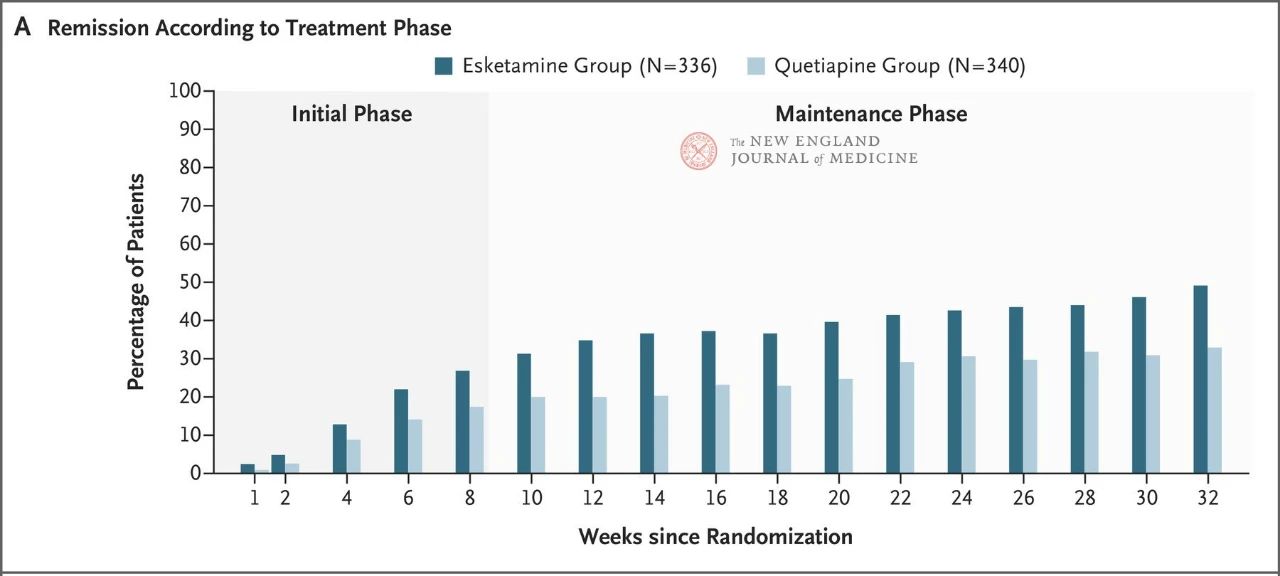
The results showed that neither drug showed particularly good efficacy, but esketamine nasal spray was slightly more effective (27.1% vs. 17.6%) (Figure 1) and had fewer adverse effects that led to discontinuation of the trial treatment. The efficacy of both drugs increased over time: by week 32, 49% and 33% of patients in the Esketamine nasal spray and quetiapine sustained-release groups had achieved remission, and 66% and 47% had responded to treatment, respectively (Figure 2). There were very few relapses between weeks 8 and 32 in both treatment groups
A striking feature of the study was that patients who dropped out of the trial were assessed as having a poor outcome (i.e., grouped with patients whose disease was not in remission or relapsed). A higher proportion of patients discontinued treatment in the quetiapine group than in the esketamine group (40% vs. 23%), a result that may reflect the shorter duration of dizziness and separation side effects associated with Esketamine nasal spray and the longer duration of sedation and weight gain associated with quetiapine sustained release.
It was an open-label trial, meaning patients knew what type of drug they were taking. The evaluators who conducted clinical interviews to determine Montgomery-Eisenberg Depression Rating Scale scores were local physicians, not remote personnel. There is a lack of perfect solutions to the serious blinding and anticipation bias that can occur in trials of drugs with short-term psychoactive effects. Therefore, it is necessary to publish data on the effects of drugs on physical function and quality of life to ensure that the observed difference in efficacy is not just a placebo effect, but also that the difference is clinically meaningful.
An important paradox of such trials is that antidepressants seem to make a sudden deterioration in mood and increase suicidal tendencies in a small number of patients. SUSTAIN 3 is a long-term, open-label extension study of the Phase 3 trial SUSTAIN, in which a cumulative follow-up of 2,769 patients – 4.3% were found to have experienced a serious psychiatric adverse event after years. However, based on data from the ESCAPE-TRD trial, a similar proportion of patients in the esketamine and quetiapine groups experienced serious adverse psychiatric events.
Practical experience with esketamine nasal spray is also encouraging. Cystitis and cognitive impairment remain theoretical rather than actual risks. Similarly, since nasal sprays must be administered on an outpatient basis, overuse can be prevented, which also improves the chances of regular review. To date, the combination of racemic ketamine or other drugs that may be abused during the use of esketamine nasal spray is uncommon, but it is still wise to monitor this possibility closely.
What are the implications of this study for clinical practice? The most important message is that once a patient does not respond to at least two antidepressants, the likelihood of achieving complete remission within two months with the addition of treatment drugs remains low. Given the desperation of some patients and their resistance to drugs, confidence in treatment can be easily undermined. Does a person with major depressive disorder respond to medication? Is the patient medically unhappy? This trial by Reif et al. highlights the need for clinicians to demonstrate optimism and tenacity in their treatment, without which too many patients are undertreated.
While patience is important, so is the speed with which the depressive disorder is addressed. Patients naturally want to recover as quickly as possible. Since the patient’s chance of benefit gradually decreases with each antidepressant treatment failure, consideration should be given to trying the most effective treatment first. If the only determinants of which antidepressant to choose after two-drug treatment failure are efficacy and safety, then the ESCAPE-TRD trial would reasonably conclude that esketamine nasal spray should be preferred as a third-line therapy. However, maintenance therapy with esketamine nasal spray usually requires weekly or twice-weekly visits. Therefore, cost and inconvenience are likely to be decisive factors affecting their use.
Esketamine nasal spray won’t be the only glutamate antagonist to enter clinical practice. A recent meta-analysis suggests that intravenous racemic ketamine may be more effective than esketamine, and two large head-to-head trials support the use of intravenous racemic ketamine later in the treatment path as an option for patients requiring electroconvulsive therapy. It seems to help prevent further depression and take control of the patient’s life.
Post time: Oct-08-2023